饲养管理
猪群规模对猪群健康的影响(二)
译者的话
猪群规模对猪群健康的影响(二)
Empirical and theoretical evidence for herd size as a risk factor for swine diseases Part 2
对于度量猪群规模的讨论,需参考一个地区的猪群(猪只)密度,或猪群内的饲养密度,因为猪群密度可能与猪群规模正相关。某些国家(如,丹麦)将较大猪群(和猪只)密度与较大猪群规模相混杂(Flori等,1995),进而阐述猪群规模效应。关于猪伪狂犬病,除考虑猪群规模为风险因素外,还需着重考虑区域内受感染的猪群或猪只密度,现今至少有五项研究证实这一观点(Austin等人,1993;Leontides等人,1994a,1995; Boelaert等,1999;Maes等,2000)。研究表明(Leontides等,1994b),将相关管理变量和猪群密度纳入统计模型后,猪群规模效应确实不再显著。
A discussion of herd size measures would be incomplete without reference either to herd (and pig) density in an area, which might also be positively correlated with herd size, or to pig stocking density within herds. Greater herd (and pig) density may be confounded with larger herd size at least in some countries, such as Denmark (Floriet al., 1995), and therefore might in part explain the reported herd-size effects. The importance of considering the density of infected herds or pig density on an area basis in addition to herd size as risk factors for pseudorabies was recognized in at least five studies (Austinet al., 1993; Leontideset al., 1994a, 1995; Boelaertet al., 1999; Maeset al., 2000). Indeed, in one study (Leontideset al., 1994b), the effect of larger herd size was no longer evident after related management variables and density of pigs had been included in the statistical models.
很少将大栏和空间的饲养密度作为引发猪病的风险因素进行研究,其原因有很多。首先,很难精确测量猪只密度,从分娩到育肥,数值会随着不同生产阶段发生改变。其次,即使存在较好的度量标准,也仅适用于大栏内,而度量猪群规模时,需要对群集或过度分散进行调整,所以研究分析会很复杂。再次,猪群规模与猪只密度之间存在共线性。在14篇有关胸膜炎或肺炎病变的研究中(表1:7位作者研究同时两种病变类型),只有7篇(50%)在度量猪群规模时对饲养密度进行测量,从而对控制猪群的肺炎问题提出标准建议(Done,1991年),强调最多改变20个环境变量,包括密度、空间和每栋猪舍内的猪数量。近期研究再次提出,对于育肥猪的呼吸道健康,空间和地面的饲养密度是重要的预测因素(Buddle等,1997;Stark等,1998;Maes等,2001)。
Pen and air-space stocking density have been investigated infrequently as risk factors for swine diseases, and there a number of possible reasons for this. First, pig density is difficult and tedious to measure accurately, and the value changes with the stage of production in farrow-to-finish herds. Secondly, even if a good measure exists, measurement is usually done at the pen level and herd-level analysis is complicated by the need to correct for clustering or over-dispersion. Thirdly, there is collinearity between herd size and pig density. Of the 14 unique reviewed studies of pleuritis or pneumonia lesions (Table 1: seven authors studied both lesion types), only seven (50%) evaluated a stocking density measure of herd size, yet standard recommendations for the control of pneumonia in problem herds (Done, 1991) emphasize alteration of up to 20 environmental variables, including density, air space, and pigs per building. The importance of air-space stocking density and floor-space stocking density as important predictors of respiratory health in finisher pig has been reaffirmed in recent studies (Buddleet al., 1997; Starket al., 1998; Maeset al., 2001).
另一项与地面、空间密度和猪群规模度量有关的因素也需考虑。多变量模型中,这些度量值可能存在共线性,这就使研究人员针对猪群规模得出一两个度量值后,便不再对其进行度量。实践中,通常采用最容易获取猪群规模的度量方式(例如,母猪数量或每年育肥猪出栏量),且无论选择哪种度量方式,作者均可以假设变量间的相关性足够高,能够相互代替。不过,后一种假设不一定正确。例如,笔者估算了三种常用的猪群规模度量方式之间的两两相关系数:作为全国养猪调查的一部分,调查了美国1306个分娩至育肥式猪场,统计其母猪数量、每年生猪销售量和总存栏量(美国农业部:动植物卫生检疫局:兽医处,1992年)。估算范围在0. 20至0.61,以此表明仅为中等线性关系。因此,多变量模型中,可采用多种猪群规模度量方法。
Another consideration relevant to the measurement of floor and air-space density and measures of herd size warrants comment. The possible collinearity of these measures in multivariable models may have dissuaded researchers from recording more than one or two measures of herd size. In practice, the most easily acquired measure of herd size (e. g. the number of breeding females or the number of finishers slaughtered per year) is generally used and, for whatever measure is selected, authors probably assume that the variables are sufficiently highly correlated to be interchangeable. The latter assumption may not necessarily be correct. For example, we estimated pairwise correlation coefficients between three frequently used measures of herd size: the number of breeding females, the number of pigs sold per year and the total inventory of pigs for 1306 US farrow-to-finish farms studied as part of the National Swine Survey (United States Department of Agriculture: Animal and Plant Health Inspection Service: Veterinary Services, 1992). The estimates ranged from 0. 20 to 0.61, which indicated only low to moderate linear relationships. Hence, multiple measures of herd size might be included in multivariable models.
猪群规模影响疾病产生的生物学原因
Biological reasons for an effect of herd size on disease occurrence
猪群外部引入病原风险更大
Greater risk of introducing infectious agents form outside the herd
引入病原携带猪
Introduction of infectious agents with carrier pigs
P=1-(1-p)n (1)
Thorburn等人(1991)建议对公式1作出修正,使其包含未替换的样本,且可对公式进一步修正,即在感染率不同的猪群中对不同数量的猪进行抽样。以下不再赘述此类修正。另外,为与下一部分的模型进行直接比较,假设病原携带猪引入受感染猪群后,病原就会在该猪群内传播。
Thorburnet al.(1991) suggested a modification of equation 1 to include sampling without replacement, and the equation could be further modified to include sampling of variable numbers of pigs from herds with different prevalences of infection. For simplicity, we do not present these modifications here. Also, to allow direct comparison with the model in the following section, we assume that, once an infectious agent is introduced into a herd with an infected pig, it is transmitted to other pigs in the same herd.
空气传播引入病原
Introduction of infectious agents transmitted by airborne routes
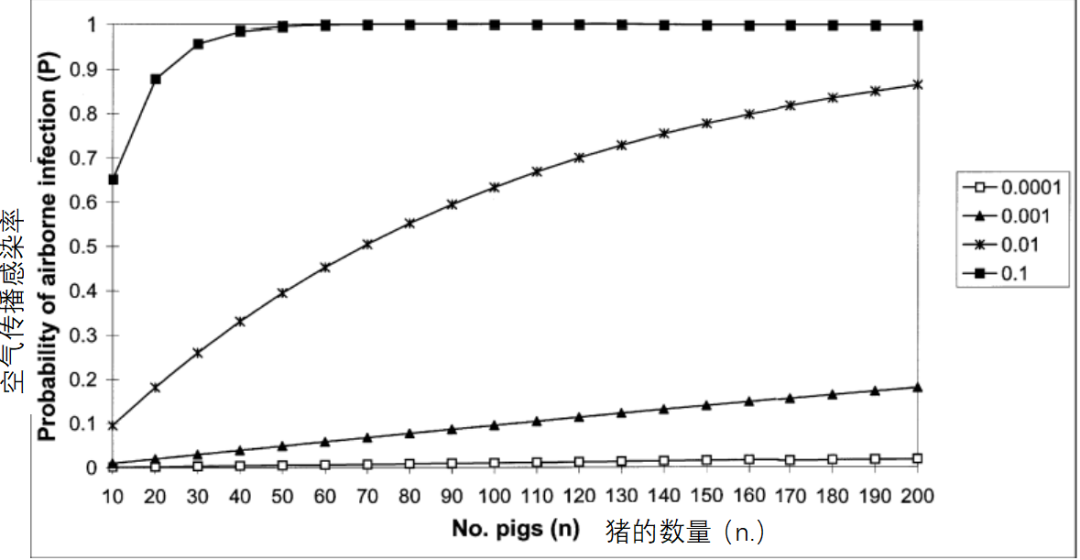
以实验为依据,假设猪群规模效应不再与猪群密度混杂,建议修正病原携带猪引入疾病的风险模型(公式1)中p和n的含义,从而将其应用于通过空气传播的病毒性疾病(Willeberg等,1994)。空气传播的情况下,P仍表示猪群被感染的概率,p指该猪群中单个猪只暴露于含有恒定病毒滴度(病毒数量/m3)的飞沫时被感染的概率,n是猪群暴露于病毒时的规模(Willeberg等,1994)。将猪群规模效应的意义概念化,可以认为猪群越大,占用的土地面积越大。假设每平方米占地面积的猪只密度固定,则500头规模育肥猪的占地面积是100头育肥猪所占面积的五倍。
On the basis of this empirical evidence and the assumption that the herd-size effect was not confounded by herd density, we proposed that the model specified for the risk of disease introduction associated with carrier pigs (equation 1) could be applied to the airborne spread of viral diseases with modification of the meanings ofpandn( Willeberget al., 1994). In the context of airborne spread,Premains the probability of a herd becoming infected,pis the probability of an individual pig in the herd becoming infected if exposed to droplet nuclei containing virus of constant concentration (virus/m3) andnis the herd size at the time of exposure to the virus (Willeberget al., 1994). One way to conceptualize the meaning of the herd-size effect is to consider that larger herds occupy a greater area of land. For example, assuming a fixed density of pigs per m2of floor space, a herd of 500 fattening pigs would occupy an area five times greater than a herd of 100 fattening pigs.
猪群被感染的概率(P)随着n和p非线性增加,但是当p足够小的时候,则是近似线性,即P= 1-(1-p)n≌np(如图1所示;p0.001时)。对于规模为n1和n2的两个猪群,每头猪感染风险为常数p,发生比(OR)为两个猪群受感染的比率,即OR = n1/ n2(上面提到的两个育肥猪群OR = 5)。在感染的ln(OR)对数模型中,存在对数转换。
The probability of the herd becoming infected (P) increases non-linearly withnandp, but whenpis small it can be shown that the relationship is approximately linear, i.e.P= 1-(1-p)n≌np (Fig.1; e.g. when p0.001). For two herds of sizen1andn2with constant riskpof infection on a per-pig basis, the odds ratio (OR) of becoming infected is the ratio of the two herd sizes, i.e. OR =n1/n2(OR = 5 for the two fattening herds referred to above). In a logistic model of the ln(OR) of infection, logarithmic transformation yields.
ln(OR)= ln(n1)- ln(n2)(2)
现场数据用于逻辑回归分析,根据猪群规模变量(X)对ln(OR)进行建模,如下所示:
Field data are used in logistic regression analysis to model ln(OR) as a function of the herd-size variable(X) as follows:



相关话题
相关文章
-
猪群规模对猪群健康的影响(二)
饲养管理 2021-12-09 -
秋冬季育肥猪饲养关键在哪里?
饲养管理 2021-12-09 -
母猪总是不吃食咋回事?如何解决?
饲养管理 2021-12-09 -
立秋后,养猪需要注意的那些事儿
饲养管理 2021-12-09 -
全价饲料就是不缺营养?
饲养管理 2021-12-09 -
母猪奶水不足怎么办?五个举措帮你化解难题
饲养管理 2021-12-09 -
怎样提高分娩舍母猪采食量?这个太重要了!
饲养管理 2021-12-09 -
仔猪7天补料做不到?这几个补料小妙招,操作简单见效快
饲养管理 2021-12-09 -
母猪奶水好坏的如何判断,该怎样改善?
饲养管理 2021-12-09 -
仔猪饲养秘籍:养猪户必读,建议收藏
饲养管理 2021-12-09 -
老王做了一个有趣的实验,告诉你母猪奶水好的秘密
饲养管理 2021-12-09 -
夏季猪厌食严重,学会这几招让你的猪胃口大开
饲养管理 2021-12-09 -
夏季过去,秋冬季养猪需要注意的8件事!
饲养管理 2021-12-09 -
断奶仔猪饲养管理四项要素,把握好可有效提高养殖效益!
饲养管理 2021-12-09 -
糖在畜禽养殖中的保健与营养性添加剂作用
饲养管理 2021-12-09
